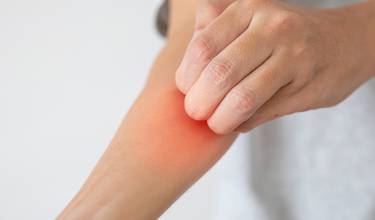
Disease information
Eczema (atopic dermatitis) is characterised by intense, chronic itch, dry skin, skin lesions and, at later stages, thickening of the skin and an increase in skin markings. It is a common skin disease that affects a large percentage of population worldwide. Eczema is a chronic inflammatory skin disease that usually begins in childhood and is often inherited.
The condition often relapses, meaning symptoms will improve for a while but then return. Although not life-threatening, eczema is associated with a significant reduction in quality of life for patients. In particular, the intense chronic itch and skin discomfort can lead to sleep disturbance, diminished self-esteem, anxiety, depression and poor performance at school and work.
Eczema may require treatment for months or years.
The treatment options include:
- Reductions of exposure to trigger factors
- Regular emollients (moisturisers)
- Intermittent topical corticosteroids
In some cases, management may also include one or more of the following:
- Antibiotics
- Antihistamines
- Phototherapy
- Corticosteroids
Therapeutic background
Many people with eczema improve their condition and relieve their symptoms using moisturising treatments and drugs such as steroid cream, however, long term use can be damaging to the skin and some people may not wish to use them regularly due to this concern.
Due to limited options for patients with mild to moderate eczema, there is a need for new and improved treatment. If you are interested in participating in a clinical trial, you will have the opportunity to test a new treatment that may have the potential to improve your condition. If you are interested and qualify to participate in a study, you may receive placebo instead of the investigational medication. A Placebo is a treatment provided instead of the investigational medication and contains no active substances. The use of placebo is standard practice in clinical studies investigating the effect of new medications and is used as a measure of comparison. Your chance of receiving placebo will be explained by study staff in advance of your participation.
Please note, participating in a clinical study and receiving placebo is greatly contributing to the clinical research. In case you receive placebo and due to your condition, you find it difficult to continue taking part in the study, you have the right to withdraw from the dosing and the study team will follow you up as appropriate. Hereafter, you will be able to use your own eczema medicine.
Who can participate?
- Male and female
- Age between 18–65 years old
- Diagnosed with eczema for at least 6 months
- BMI between 18–35, minimum weight of 7 stones and 12 pounds
Location(s)
United Kingdom
- Cannock
- Manchester
- Teeside
- Prescot
- Tankersley
- Blackpool
- Leeds
Compensation
You will receive up to £2,230 for taking part in this clinical research study regarding eczema. This amount of money corresponds to all study visits.
Reimbursement
Participating in the study will not entail any extra expense to your usual treatment, as the Sponsor provides the investigational drug and any tests required by the trial.
You will be paid for travel expenses as well.
Study description
Clinical research studies assess the safety and effectiveness of investigational medications in treatment of mild to moderate eczema. If you are interested and qualify to participate, staff at a study site will provide you with a detailed overview of the steps required to participate before you agree to take part. The staff will also take the time to review and discuss any questions you may have to ensure you are comfortable with participation.
The following are examples that are typical of participating in an eczema related clinical research study.
A screening assessment period
A period, which is conducted to confirm your eligibility into the research project based on clinical or laboratory assessments. For the screening session you will have to visit a site up to a month before you can receive a study medication. There will be only one screening visit, where site staff will ask you some questions about your health and will perform some tests (e.g., blood test, pregnancy test, measure body weight and height, ECG etc.).
Washout period
The washout period consists of 7 days and starts one week before you may receive a study medication. During this period, you will be asked to apply a study-supplied moisturising cream and shower cream instead of any cream you are currently using. In addition, you may not use other eczema related therapy or treatment, other than the supplied cream and shower cream during this period.
Treatment period
If you are eligible to participate in a study, you will enter a treatment period. Here you will receive either a study medication or placebo. During the treatment period, you will have to visit a site up to7 times. The maximum expected duration of participation for you is not expected to exceed approximately 11 weeks. During the treatment period, you will apply an investigative ointment or placebo ointment a maximum of three times daily. The first time you will apply an investigative ointment will be on site, followed by home administration. Site staff will provide you all information about how and when to apply the ointment.
Your health will be closely monitored by study staff throughout your participation in a clinical research study. You can withdraw your participation at any time.